
Application of chlorinated rubber in adhesives
2025-01-18
Chlorinated rubber is a rubber derivative obtained by chlorinating natural rubber or synthetic polyisoprene rubber. The chlorination process involves complex reactions such as addition, substitution, and cyclization, and the final product is an irregular cyclic polymer [1] with a chlorine content of about 65%, and an empirical formula of (C10H11Cl7)n. Due to the strong polarity and chemical inertness of the C–Cl bond, chlorinated rubber has good chemical corrosion resistance, wear resistance, flame retardancy, water resistance, and adhesion. It has been widely used in the field of anti-corrosion coatings, including ship paints, road marking paints, printing inks, building coatings, and offshore oil platform coatings [2]. In fact, chlorinated rubber has good adhesion to both metals and polymers, and has broad application prospects as an adhesive. Currently, there is little research on chlorinated rubber adhesives, and their applications are mainly found in patent literature.
1. Properties of Chlorinated Rubber
Chlorinated rubber has a strong polar molecular structure, but good water resistance, with a water absorption rate of only 0.1%~0.3% [2]; at the same time, the molecular chain contains a rigid cyclic structure, making movement more difficult, resulting in lower water vapor and oxygen permeability than most polar polymers (such as acrylates, epoxy resins, and alkyd resins), only one-tenth of that of alkyd resins [3]. Therefore, corrosive media such as water and oxygen are difficult to penetrate the chlorinated rubber film to corrode base materials such as steel. As an adhesive, it can also prevent water vapor from damaging the adhesive interface.
Due to its high chlorine content, chlorinated rubber has high intrinsic cohesive energy, and the resulting film is brittle. Therefore, plasticizers are usually added to improve its brittleness. Commonly used plasticizers include chlorinated paraffin wax, chlorinated biphenyl, or phthalate esters [4, 5]. Chlorinated rubber has good adhesion to polar interfaces such as metals. However, for some less polar interfaces (such as natural rubber and styrene-butadiene rubber), its strong polarity is detrimental to adhesion. Therefore, depending on the properties of the material to be bonded, it is often necessary to use some polymers to achieve bonding between metals and different materials. Chlorinated rubber can be used with chloroprene rubber, nitrile rubber, phenolic resin, alkyd resin, acrylic resin, polyurethane, etc. [4, 6–10] to prepare different adhesives. Chinese patent CN 101418197 A discloses a chloroprene rubber adhesive modified with chlorinated rubber, in which the addition of chlorinated rubber enhances the adhesion to polar surfaces such as metals [6]. According to US Patent US 4,256,615 [8], adding an appropriate amount of chlorinated rubber to a two-component castor oil polyurethane adhesive can significantly improve the initial adhesion strength, and the ultimate strength is also improved.
Like other chlorinated polyolefins, chlorinated rubber is prone to losing hydrogen chloride at high temperatures. Thermal degradation studies have shown that between 160~390 °C, chlorinated rubber loses hydrogen chloride, forming a conjugated structure and turning yellow; between 390~585 °C, chlorinated rubber mainly undergoes oxidative degradation [11]. In a humid and hot environment, it starts to decompose at 60 °C [3]. Therefore, chlorinated rubber products must contain heat stabilizers to absorb the trace amount of hydrogen chloride decomposed to prevent further catalysis of the dechlorination process. Commonly used heat stabilizers include lead soaps, epoxides, organic phosphites, and aliphatic polyamines [4, 12]. With suitable stabilizers, chlorinated rubber can be used in heat-resistant adhesives.
2. Interaction between Chlorinated Rubber and Metal Interfaces
A major application area for chlorinated rubber as an adhesive is the bonding between metals and rubber. Therefore, understanding the interaction between chlorinated rubber and metal interfaces is of great significance for designing adhesive formulations. Feliu S et al. studied the interaction of a commercial chlorinated rubber paint (dissolved in xylene, containing alumina and silica fillers, specific formulation unknown) with metal interfaces such as copper, aluminum, stainless steel, cold-rolled steel, and zinc [13]. The study found that chlorinated rubber has the strongest adhesion to polyvinyl chloride, and no chemical changes were found at the interface. Metallic zinc can degrade C–Cl to form ZnCl2, resulting in the lowest bonding strength. For copper and cold-rolled steel, some Cl− is formed at the interface, resulting in a weak hydrocarbon interface layer, but its bonding strength is higher than zinc. For aluminum and stainless steel, due to the easy formation of a passivation layer (Al2O3 or a chromium protective layer) on their surface, the amount of Cl− generated by degradation is small, so their bonding strength is higher. Liu XW et al. studied the anti-corrosion effect of different coating systems on carbon steel, and found that the zinc-rich primer/chlorinated rubber topcoat system exhibited more severe corrosion than the chlorinated rubber coating alone [14], which may be due to the degradation of chlorinated rubber by zinc. Adding an epoxy intermediate coat between the two layers of coating greatly improves the corrosion resistance. This shows that metallic zinc has a greater destructive effect on chlorinated rubber coatings, while chlorinated rubber has better adhesion to inert interfaces such as aluminum and stainless steel.
Gao Shouchao et al. used X-ray photoelectron spectroscopy to study the elemental composition of the interface between chlorinated rubber and metal, and found that the adhesion between the two is due to van der Waals attraction, and no chemical bonding was produced [15]. Increased surface roughness is beneficial to adhesion, because it increases the contact area between the two phases [16]. Some studies have also shown that the rust layer on the steel surface can degrade chlorinated rubber, forming Cl−, which in turn catalyzes the degradation of the coating [17]. Humid environments accelerate degradation [18], and in practical applications, the addition of fillers such as glass flakes can greatly reduce the water vapor permeability of the coating. Berio M et al.'s research showed that the addition of zinc phosphate and other anti-corrosion pigments actually reduces the water absorption rate of the coating [19]. In addition, many anti-corrosion pigments, such as aluminum phosphate and zinc phosphate, can form dense passivation films on the steel surface [3], thereby reducing the degradation effect of the metal on chlorinated rubber. However, there is little research on the interaction between chlorinated rubber and metal interfaces in such complex systems containing anti-corrosion pigments.
3. Chlorinated Rubber Adhesives
3.1 Applications in Metal-Rubber Bonding
The bonding of metal and rubber is widely used in aerospace, automotive, and other fields. Examples include engine oil seals and flexible joints in solid rocket engine nozzles, all of which involve bonding metal and rubber. Chlorinated rubber, due to its excellent chemical inertness, can meet the requirements of oil-resistant and high-temperature adhesives through appropriate formulation design.
Chinese patent CN 101421370 A discloses an oil-resistant and high-temperature adhesive used for bonding acrylic rubber and degreased cold-rolled steel plates, mainly used for oil seals in engines and transmission systems [20]. This adhesive is formed by dispersing 45~75% (by weight, the same below) phenolic resin, 5~25% chlorinated rubber, and 10~30% metal oxides (zinc oxide and titanium oxide) in a solvent. If the amount of chlorinated rubber in the formulation is reduced or absent, the adhesion to the metal is insufficient, and peeling is easy; if the amount of chlorinated rubber is excessive, the adhesion to the acrylic rubber is insufficient, and the interface is also prone to peeling. By adjusting the ratio of the two polymers, a bonded interface with a bonding strength greater than that of the acrylic rubber itself can be obtained, and the fracture interface can be 100% covered by acrylic rubber.
US Patent 2,459,742 discloses a nitrile rubber-metal adhesive [21], which is characterized by the addition of 1~5% by weight of aliphatic polyamine (tetraethylenepentamine, diethylenetriamine, triethylenetetramine, etc.) mixed with chlorinated rubber solution. After curing, its bonding strength did not decrease even after immersion in gasoline for more than 10 weeks. The polyamine absorbs and decomposes hydrogen chloride and crosslinks the chlorinated rubber adhesive layer, enhancing the oil resistance of the adhesive, which can be used for bonding aircraft fuel tanks and metal parts.
US Patent 3,108,035 discloses a one-component chlorinated rubber solvent-type adhesive, whose solid components consist of 100 parts chlorinated rubber, 5~200 parts lead dioxide (preferably 60 parts), and 2~30 parts dibenzoquinonedioxime (preferably 10 parts) [22]. After curing at 149~177 °C, the bonding strength to rubber and 1010 steel varies depending on the type of rubber. For nitrile rubber and chloroprene rubber, a peel strength of 27 kg/cm can be produced; for natural rubber and styrene-butadiene rubber, a peel strength of 25 kg/cm can be produced; and for butyl rubber and steel, the bonding strength is 16 kg/cm. This influence of different rubbers on bonding strength can also be seen in US Patent 4,994,519, which discloses a rubber-metal adhesive whose main components are chlorinated rubber and brominated poly(dichlorobutene). In addition, p-benzoquinonedioxime and sulfur are added as vulcanizing agents [23]. The bonding strength of this adhesive to rubber and steel decreases in the order of nitrile rubber, styrene-butadiene rubber, and natural rubber. Obviously, chlorinated rubber adhesive is very good for bonding polar rubber and metal.
3.2 Applications in Fiber-Reinforced Rubber
Fiber-reinforced rubber is mainly used in transmission systems, such as synchronous belts and conveyor belts in automobile engines. Its operating temperature is high (above 100 °C), requiring the fiber-rubber adhesive used to be high-temperature resistant. In recent years, high-temperature and oil-resistant rubbers such as hydrogenated nitrile rubber and chlorosulfonated polyethylene have been widely used in the manufacture of conveyor belts. Their reinforcing fibers mainly include glass fiber, polyester, and nylon. In order to enhance the bonding strength between the fiber and the rubber, the fiber is generally subjected to three-layer treatment: first, the fiber surface is activated with an epoxy compound or isocyanate; then, a resorcinol-formaldehyde-rubber latex (RFL) emulsion is coated; finally, a chlorinated rubber adhesive is coated. The third layer of adhesive plays a bonding role.
Chinese patent CN 101671959 A discloses a treating agent for preparing glass fiber yarns for reinforced rubber (pre-treatment of fibers with RFL emulsion is required before using this treating agent) [24]. This treating agent enhances the bonding strength between chlorinated polyethylene, hydrogenated nitrile rubber, and glass fiber, and can improve the service life and heat resistance of automobile engine synchronous belts. This treating agent contains chlorosulfonated polyethylene and chlorinated rubber, as well as isocyanates, and the specific formula is shown in Table 1. As can be seen from the table, the treating agent using chlorinated rubber and chlorosulfonated polyethylene together improves the bonding strength and hot water resistance, which is better than using either one alone.
Table 1. Adhesive formulation and properties for the interface between glass fiber and hydrogenated nitrile rubber [24]
Component | Trade Name and Origin | Formulation (parts by weight) | ||
Example | Comparison 1 | Comparison 2 | ||
Zinc methacrylate |
| 0.8 | 0.8 | 0.8 |
Chlorosulfonated polyethylene | Hypalon 40 Showa Denko DuPont Company | 3 | 6 | None |
Chlorinated rubber | Superchlon Japan Paper Chemicals | 3 | None | 6 |
Isocyanate | MR-200 Japan Polyurethane Company | 1.1 | 1.1 | 1.1 |
p,p ' - Diphenylbenzoylbenzoquinone Dioxime |
| 1.5 | 1.5 | 1.5 |
The above solid parts are added to 81.6 parts of formaldehyde to form a 10% solution | ||||
Bond strength under normal conditions ( N/25 mm ) | 187 | 151 | 126 | |
100 °C Hot water treatment 1 Bond strength after hours ( N/25 mm ) | 162 | 125 | 116 | |
Table 2. Adhesive formulation and properties for the interface between polyester fiber and hydrogenated nitrile rubber [25]
Formulation Description | Peel strength [kg/2.54 cm] ( Proportion of rubber on the peeled section ) [%] | Conveyor belt test 120 °C | ||||||
Initial | 140 °C After treatment | Lifespan (h) | Cause of failure | |||||
1 Days | 3 Days | 5 Days | 7 Days | |||||
Example | Containing nitrile rubber and chlorinated rubber | 40 (80) | 40 (80) | 38 (80) | 35 (85) | 30 (90) | 220 | Rubber cracks |
Comparison 1 | Only containing nitrile rubber component | 20 (50) | 15 (50) | 12 (40) | 10 (40) | 5 (30) | 24 | Fiber peeling |
Comparison 2 | Only containing chlorinated rubber component | 40 (80) | 30 (70) | 22 (60) | 12 (50) | 10 (50) | 220 | Fiber peeling |
US Patent US 5,219,902 also discloses an adhesive for hydrogenated nitrile rubber reinforced with fibers (polyester, polyaramid, nylon, etc.) [25]. The adhesive contains (hydrogenated) nitrile rubber and chlorinated rubber (generally used in equal weight ratio) and toluene solvent (10% solids content). Before using this adhesive, the fiber needs to be isocyanate activated and coated with RFL emulsion. Table 2 shows the performance of polyester-hydrogenated nitrile rubber composite prepared with this adhesive. After treatment at 140°C for 7 days, the bonding strength is maintained well, which is better than the effect of using nitrile rubber or chlorinated rubber alone as adhesive. The conveyor belt made of this composite material shows rubber cracking after 220 hours of use in a 120°C environment. However, fiber peeling was observed in the control sample (Table 2), indicating that the bonding strength of the control sample is insufficient.
Japanese Patent JP 02258653 discloses an adhesive composed of hydrogenated nitrile rubber, chlorinated rubber and isocyanate, used to enhance the bonding between glass fiber and rubber, and used to prepare high-performance rubber products [26].
3.3 Applications in other fields
Chinese Patent CN 102153965 A discloses a room temperature curing high temperature resistant conveyor belt joint adhesive and its preparation method [27]. This adhesive uses chloroprene rubber, chlorinated rubber and isocyanate as the main film-forming substances, and can bond the lap joint of the conveyor belt at room temperature. The surface drying time is 1-10 seconds, the average shear strength is 3 MPa, and the average tensile strength is 2.5 MPa. This adhesive has good high temperature resistance. After aging at 200 °C for 12 hours, it still has a shear strength of 1.55 MPa and a tensile strength of 1.45 MPa. This adhesive can increase the heat resistance temperature of the conveyor belt joint to 180 °C.
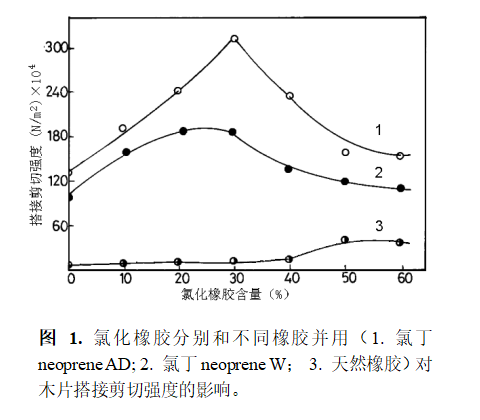
John N et al. studied rubber adhesives used for bonding wood chips [28]. The basic components are chloroprene rubber and chlorinated rubber. When the amount of chlorinated rubber is between 0 and 30%, the bonding strength increases almost linearly. When the content of chlorinated rubber is greater than 30%, the bonding strength decreases (see Figure 1). Chlorinated rubber and natural rubber are used together, the bonding strength is the lowest, and the increase of chlorinated rubber dosage has little effect on the strength change.
4. Conclusion
Chlorinated rubber has both water resistance, strong polarity and chemical inertness, and is compatible with many polar polymers. After adding appropriate heat stabilizers (to prevent dehydrochlorination), the prepared adhesive has high bonding strength, oil resistance, and high temperature resistance. Metal-rubber products and fiber-reinforced rubber products prepared from chlorinated rubber adhesives have been successfully applied in automotive oil seals, engine conveyor belts, and other fields. The current application data is mainly found in patent literature, and the influence of the properties of chlorinated rubber itself (molecular weight, molecular weight distribution, etc.) on the bonding performance has not been systematically studied. The emergence of high-viscosity chlorinated rubber (i.e., high molecular weight chlorinated rubber) may further improve its adhesion and obtain chlorinated rubber adhesives with better properties.

